Nemolizumab approved for prurigo nodularis and atopic dermatitis
In Clinical news
Follow this topic
Bookmark
Record learning outcomes
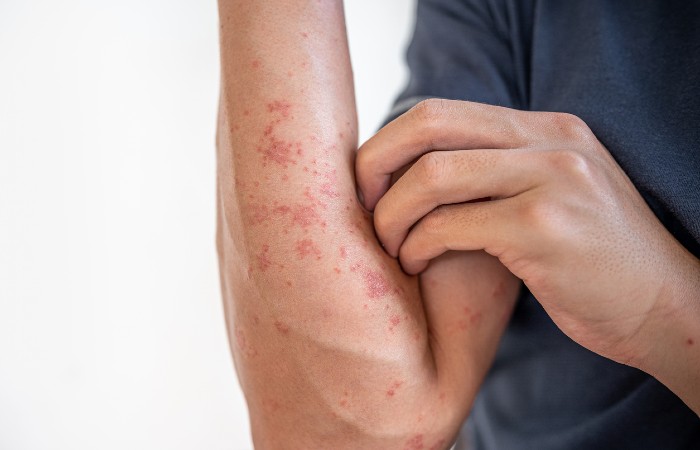
Nemolizumab has been approved by the Medicines and Healthcare products Regulatory Agency for the treatment of two skins conditions – moderate to severe prurigo nodularis for adults aged 18 and over, and moderate-to-severe atopic dermatitis for adults and adolescents aged 12 and over.
Prurigo nodularis is a chronic skin condition that causes hard, itchy bumps called nodules. The safety and efficacy of nemolizumab for this condition were demonstrated in two clinical trials in adults (aged 18 yrs and over). Its safety and efficacy have not been established in patients below the age of 18 years.
Nemolizumab (Nemluvio, Galderma) has also been approved for patients aged from 12 years and with a body weight of at least 30kg for the treatment of moderate-to-severe atopic dermatitis. It has been approved for use in combination with topical treatment when the atopic dermatitis is not well controlled by topical therapies alone.
Nemolizumab’s recommended dosage is 30 mg and it is administered as an injection in a pre-filled pen or pre-filled syringe. The most common side effects with Nemluvio in prurigo nodularis and atopic dermatitis are hypersensitivity and injection site reactions.